Integra Dermal Regeneration Template - Web integra® dermal regeneration template has two layers: Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for revascularization and neodermal formation. The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation. Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: Integra® meshed dermal regeneration template appeals packet. Web integra® dermal regeneration template and integra® meshed dermal regeneration template (integra template) are advanced bilayer matrices designed to provide immediate wound closure and permanent regeneration of dermis. Integra® dermal regeneration template materials manager cdm set up sheet. Reimbursement information request (rir) form. • exposed arteries, veins, organs, anastomotic sites or nerves • malignancy in the wound • untreated osteomyelitis Web integra® dermal regeneration template appeal packet.
Negative pressure wound therapy foam in place with con tinuous
Integra® meshed dermal regeneration template appeals packet. Integra® dermal regeneration template (idrt) 2002 pma. The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation. Integra® dermal regeneration template materials manager cdm set up sheet. Integra® dermal regeneration template (idrt) 1996 pma.
(PDF) The Use of Integra Dermal Regeneration Template Versus Flaps for
Integra® meshed dermal regeneration template (mdrt) fda approval letter. Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: • exposed arteries, veins, organs, anastomotic sites or nerves • malignancy in the wound • untreated osteomyelitis Web integra ® dermal.
Integra® Dermal Regeneration Template
Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for revascularization and neodermal formation. Integra® meshed dermal regeneration template materials manager cdm set up sheet. Web integra® dermal regeneration template has two layers: Integra® meshed dermal regeneration template (mdrt) fda approval letter. Integra® dermal regeneration template materials.
Integra® Meshed Dermal Regeneration Template
The use of integra has slowly grown and has now become an important consideration along the reconstruc. The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation. Integra® dermal regeneration template (idrt) 2002 pma. Integra® dermal regeneration template materials manager cdm set up sheet. Web when using integra meshed.
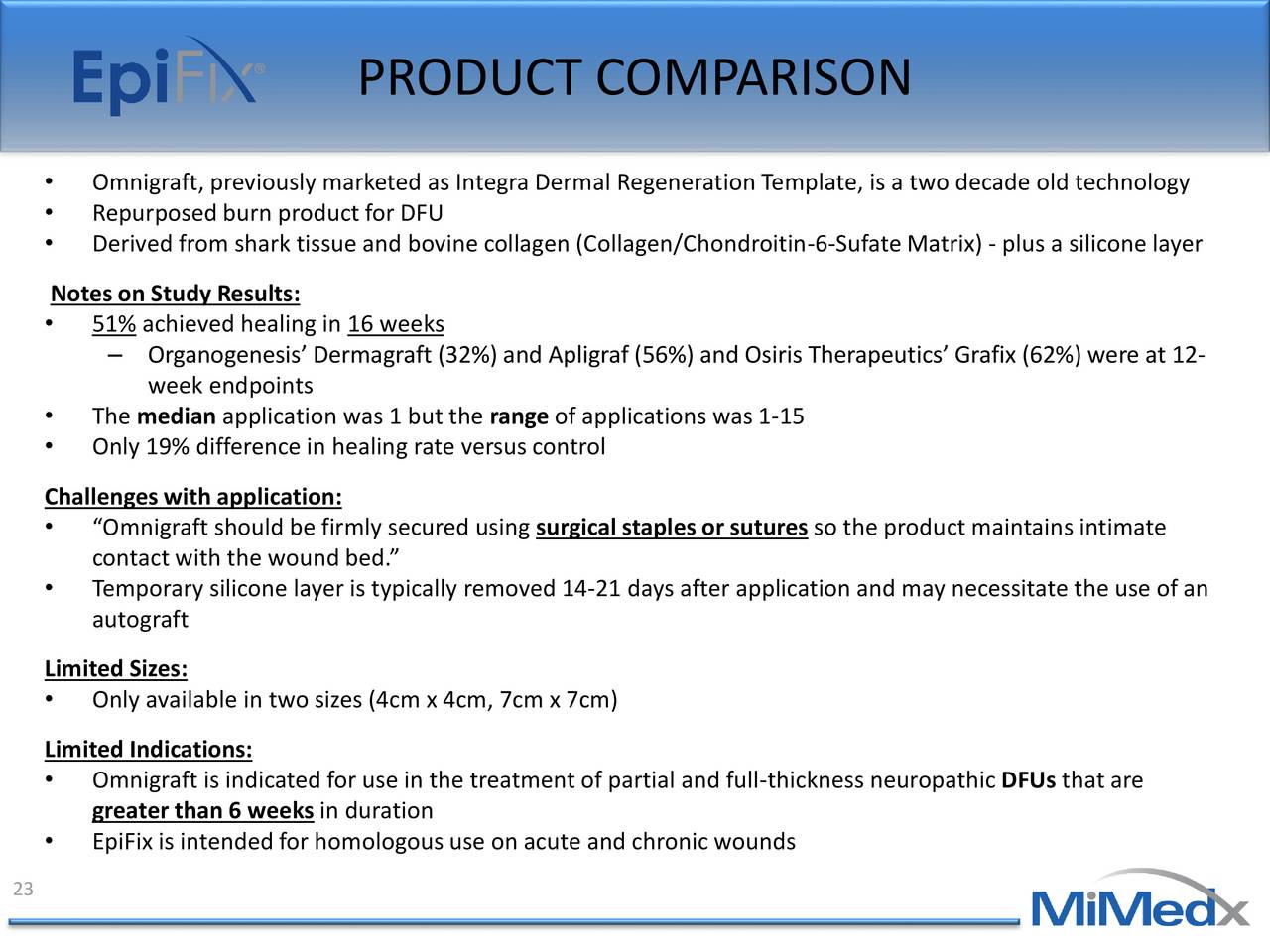
MiMedx Group (MDXG) Investor Presentation Slideshow MiMedx Group
Web integra® dermal regeneration template appeal packet. Web integra® dermal regeneration template has two layers: Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: A thick underlayer made of pure collagen (protein) from cows and a substance called glycosaminoglycan.
Extraordinary Large Giant Congenital Melanocytic Nevus Treated with
Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: Reimbursement information request (rir) form. The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation. Integra® dermal regeneration template.
Integra Dermal Regeneration Template williamsonga.us
• exposed arteries, veins, organs, anastomotic sites or nerves • malignancy in the wound • untreated osteomyelitis Reimbursement information request (rir) form. The use of integra has slowly grown and has now become an important consideration along the reconstruc. Integra® meshed dermal regeneration template (mdrt) fda approval letter. Web integra® dermal regeneration template and integra® meshed dermal regeneration template (integra.
INTEGRA DERMAL REGENERATION TEMPLATE Trademark of INTEGRA LIFESCIENCES
Web integra® dermal regeneration template has two layers: Integra® dermal regeneration template (idrt) 1996 pma. Web integra ® dermal regeneration template structure of integra template designed to promote organized regeneration of dermal tissue silicone layer • enables immediate wound closure • controls fluid loss • provides mechanical protection • provides a bacterial barrier • water vapor transmission rate similar to.
Integra Dermal Regeneration Template williamsonga.us
Reimbursement information request (rir) form. Web integra ® dermal regeneration template structure of integra template designed to promote organized regeneration of dermal tissue silicone layer • enables immediate wound closure • controls fluid loss • provides mechanical protection • provides a bacterial barrier • water vapor transmission rate similar to that of normal skin Integra® dermal regeneration template (idrt) 1996.
(PDF) Integra®Dermal Regeneration Template and SplitThickness Skin
Web integra ® dermal regeneration template structure of integra template designed to promote organized regeneration of dermal tissue silicone layer • enables immediate wound closure • controls fluid loss • provides mechanical protection • provides a bacterial barrier • water vapor transmission rate similar to that of normal skin Integra® meshed dermal regeneration template (mdrt) fda approval letter. Reimbursement information.
Integra® dermal regeneration template (idrt) 1996 pma. Web integra ® dermal regeneration template structure of integra template designed to promote organized regeneration of dermal tissue silicone layer • enables immediate wound closure • controls fluid loss • provides mechanical protection • provides a bacterial barrier • water vapor transmission rate similar to that of normal skin Integra® meshed dermal regeneration template materials manager cdm set up sheet. • exposed arteries, veins, organs, anastomotic sites or nerves • malignancy in the wound • untreated osteomyelitis Reimbursement information request (rir) form. Web integra® dermal regeneration template appeal packet. Integra® meshed dermal regeneration template appeals packet. Web integra® dermal regeneration template has two layers: Reimbursement information request (rir) form. Web integra ® dermal regeneration template is a bilayer membrane system for skin replacement. The use of integra has slowly grown and has now become an important consideration along the reconstruc. Integra® dermal regeneration template materials manager cdm set up sheet. Integra® meshed dermal regeneration template (mdrt) fda approval letter. Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: Web integra® dermal regeneration template and integra® meshed dermal regeneration template (integra template) are advanced bilayer matrices designed to provide immediate wound closure and permanent regeneration of dermis. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for revascularization and neodermal formation. A thick underlayer made of pure collagen (protein) from cows and a substance called glycosaminoglycan made from shark cartilage. Integra® dermal regeneration template (idrt) 2002 pma. The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation.
Reimbursement Information Request (Rir) Form.
The use of integra has slowly grown and has now become an important consideration along the reconstruc. Web integra ® dermal regeneration template is a bilayer membrane system for skin replacement. Web integra® dermal regeneration template appeal packet. Integra® dermal regeneration template (idrt) 1996 pma.
Integra® Meshed Dermal Regeneration Template (Mdrt) Fda Approval Letter.
Web when using integra meshed dermal regeneration template with negative pressure wound therapy, follow contraindications for the specific negative pressure wound therapy device utilized, such as in the presence of: Integra® meshed dermal regeneration template materials manager cdm set up sheet. Integra® dermal regeneration template materials manager cdm set up sheet. Reimbursement information request (rir) form.
Web Integra ® Dermal Regeneration Template Structure Of Integra Template Designed To Promote Organized Regeneration Of Dermal Tissue Silicone Layer • Enables Immediate Wound Closure • Controls Fluid Loss • Provides Mechanical Protection • Provides A Bacterial Barrier • Water Vapor Transmission Rate Similar To That Of Normal Skin
Web integra® dermal regeneration template and integra® meshed dermal regeneration template (integra template) are advanced bilayer matrices designed to provide immediate wound closure and permanent regeneration of dermis. Web first developed for coverage of burn wounds, integra (integra lifesciences) is a synthetic acellular dermal regeneration template that provides a base for revascularization and neodermal formation. Integra® meshed dermal regeneration template appeals packet. Web integra® dermal regeneration template has two layers:
• Exposed Arteries, Veins, Organs, Anastomotic Sites Or Nerves • Malignancy In The Wound • Untreated Osteomyelitis
The dermal replacement layer (collagen and glycosaminoglycan) is designed with a controlled porosity and defined degradation rate to promote neodermal formation. Integra® dermal regeneration template (idrt) 2002 pma. A thick underlayer made of pure collagen (protein) from cows and a substance called glycosaminoglycan made from shark cartilage.